Biocomposites invests in Renovos Biologics –
developers of the RENOVITE® nanoclay therapeutic delivery platform
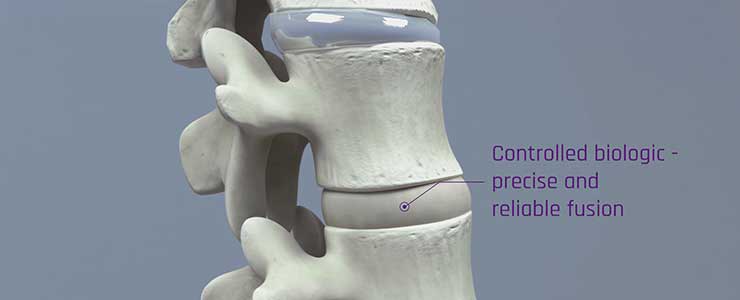
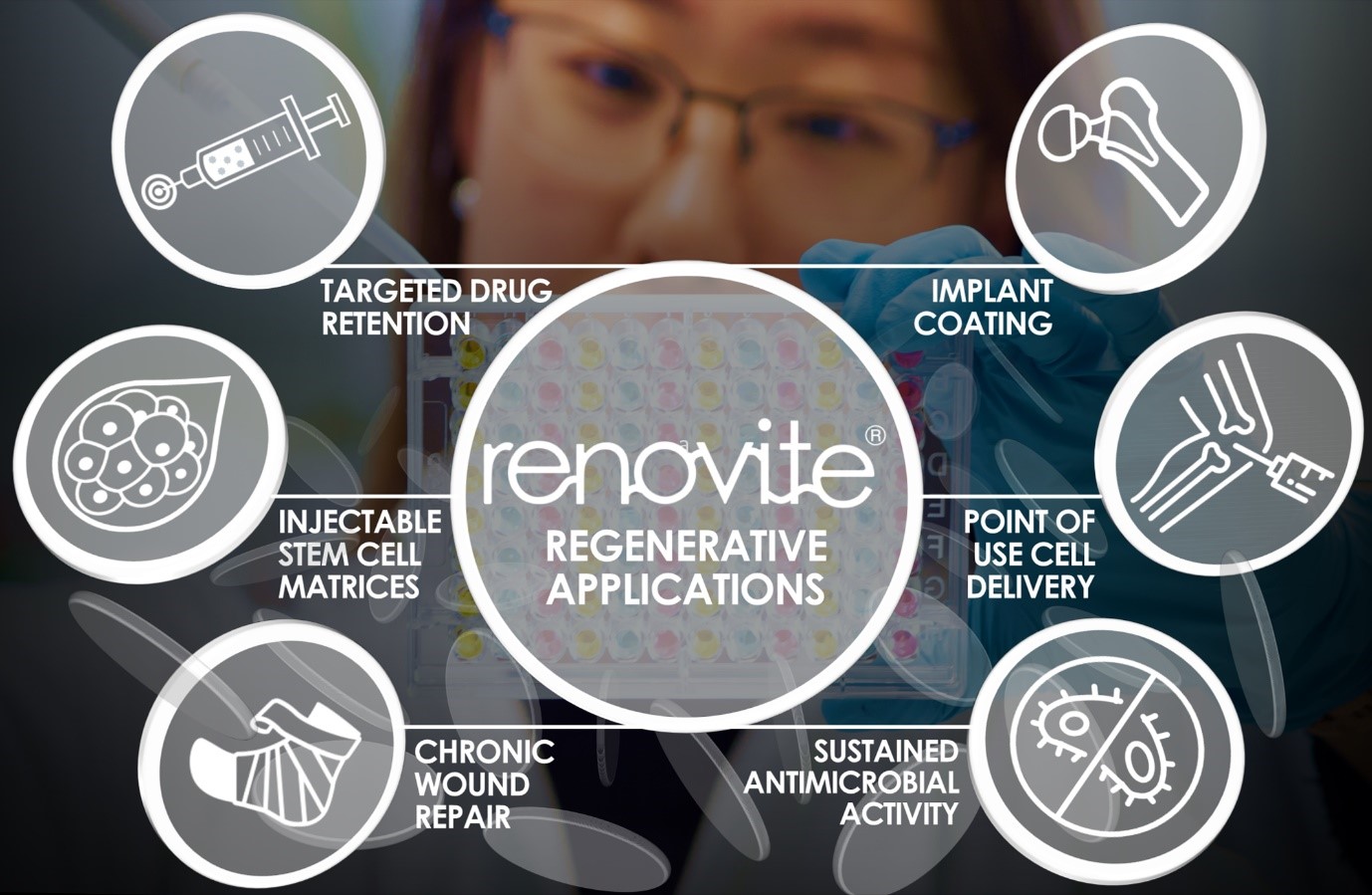
RENOVITE ® nanoclay is a novel therapeutic delivery platform for precision regenerative medicine
Investment will help fund pre-market approval for use in spine, trauma and orthopaedics
Biocomposites, CEO, Michael Harris will join Renovos Biologics Board of Directors
Keele, UK, 5 July 2023 – Biocomposites, an international medical devices company that
engineers, manufactures and markets world leading products for regenerating bone and
managing infection in bone and soft tissue, today announces it has taken a minority-share
interest in Renovos Biologics (Renovos), an innovative biologics company born out of
research into the properties of nanoclay at the University of Southampton, UK. Michael
Harris, CEO, Biocomposites will join Renovos Biologics Board of Directors.
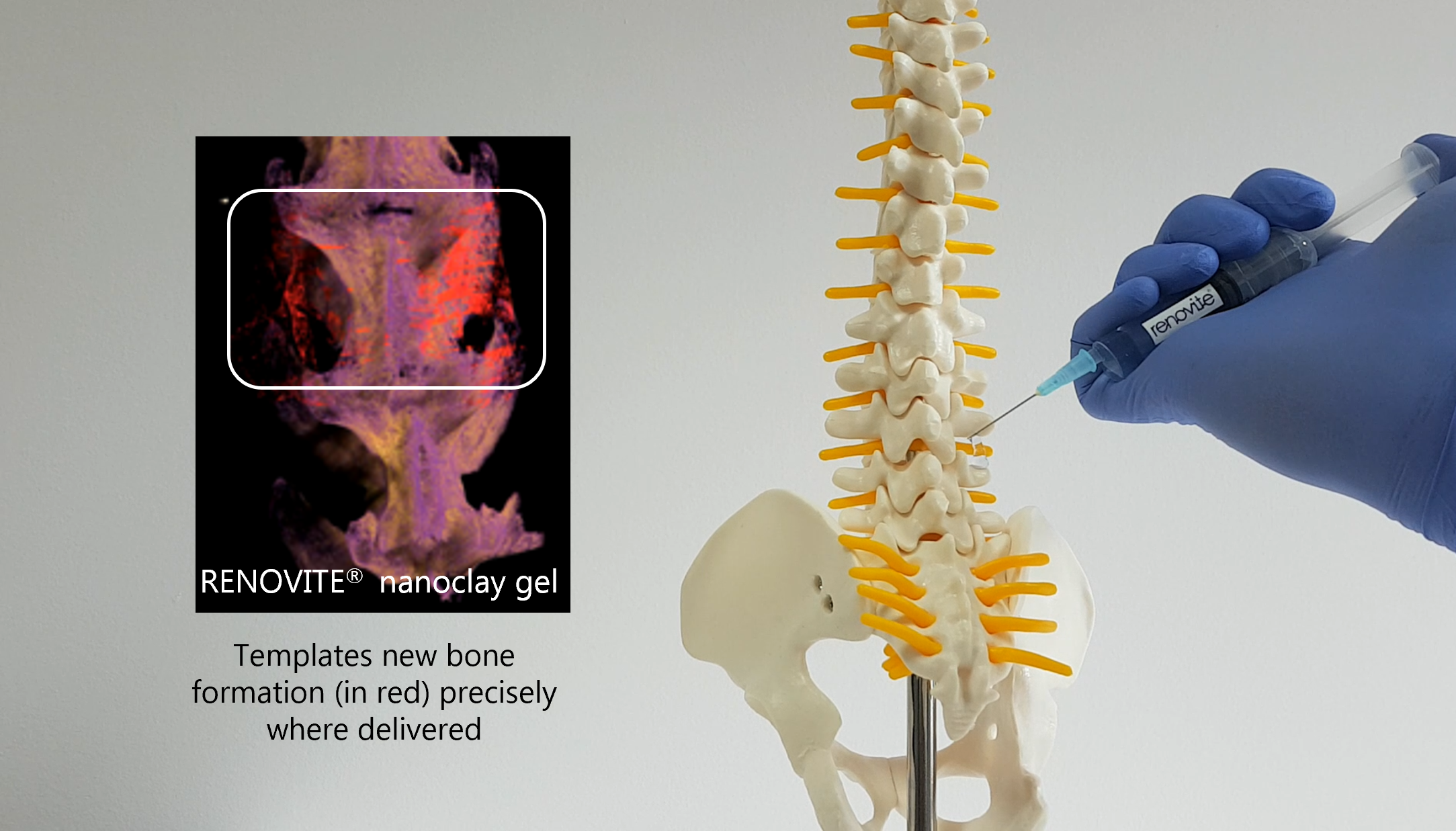
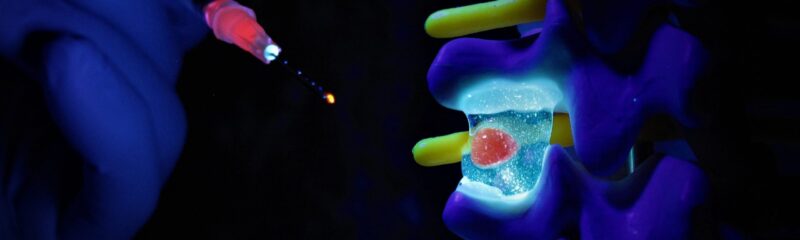
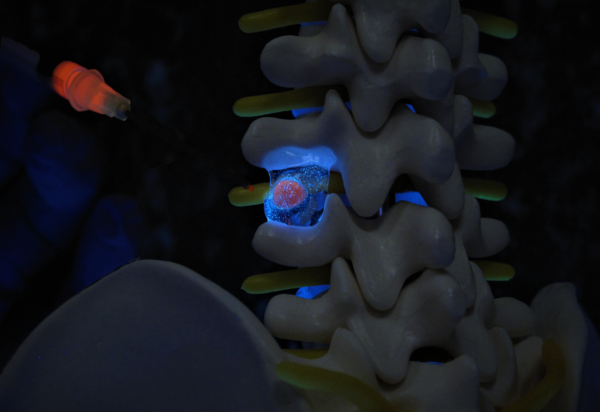
Renovos’ lead product RENOVITE® is a synthetic, biodegradable nanoclay carrier that can be
used to place a wide range of pharmaceutical drugs and biologics at the site of treatment. It
can be injected through a 23-gauge cannula in support of minimally-invasive procedures and
sets into a stiff gel upon contact with physiological fluids, such as blood serum. As a cell-
responsive carrier it requires a lower dose of therapeutic drug to accelerate healing.
The investment from Biocomposites will allow Renovos to access Biocomposites’ expertise
and know-how in the development of drug carriers to progress RENOVITE® to pre-market
approval as a carrier for use in spine, trauma and orthopaedics. And in due course, provide
Renovos access to Biocomposites’ established, global distribution network.
Michael Harris, Chief Executive Officer of Biocomposites, commented: “The potential for
RENOVITE® as a next generation drug carrier that can enhance the activity of therapeutic
drugs at lower doses, whilst giving the surgeon much greater freedom to use in minimally
invasive procedures, is very exciting. Following on from our recent acquisition of Artoss in
June this year, and Subiton and Synimed last year, Biocomposites has established itself as
the go-to provider for surgeons requiring bone regeneration and/or managing infection in
bone and soft tissue.”
Dr Agnieszka Janeczek, Chief Executive Officer of Renovos Biologics added: “We have long
believed in the potential of RENOVITE® to overcome the challenges in tissue regeneration by
providing unprecedented retention of therapeutics at the site of repair. With the
opportunity to partner with Biocomposites, Renovos now have access to their deep
knowledge and expertise in drug developments, as well as their global distribution network
as products come to market.”
Contact for media enquiries:
Optimum Strategic Communications
Hollie Vile, Charlotte Hepburne-Scott, Zoe Bolt, Katie Flint
Tel: +44 (0)20 3882 9621
biocomposites@optimumcomms.com
About Biocomposites
Biocomposites is an international medical device company that engineers, manufactures
and markets world leading products for use in infection management in bone and soft
tissue. Based in Keele, UK, it has global operations across Europe, USA, Canada, China and
India. Biocomposites is a world leader in the development of innovative calcium compounds
and polymers for surgical use. Its products regenerate bone and target infection risks across
a variety of specialties, including musculoskeletal infection, orthopaedics, trauma, spine,
foot and ankle, podiatry and sports injuries. Biocomposites products are now used in over
120,000 procedures per annum and sold in more than 40 countries around the world.
Following the acquisition of Artoss GmbH, Biocomposites has acquired proprietary
NanoBone technology to its product portfolio, a combination of nanocrystalline
hydroxyapatite and silica gel – to provide optimal bone formation with easy handling.
Please visit biocomposites.com to learn more.
About Renovos Biologics
Renovos is a regenerative medicine company and a specialist developer of RENOVITE®
synthetic nanoclay for medical use. Stemming from research at the University of
Southampton, UK, Renovos’ proprietary RENOVITE® technology greatly improves the
performance of a range of regenerative medicine products, with first targets in
orthobiologics. RENOVITE® presents a novel mechanism of action, offering unprecedented
retention of therapeutic agents at the target site of repair, mitigating their effects to a
precisely controlled area, creating a step-change improvement in their efficacy, safety and
ease of use, in biodegradable and injectable formulations, suitable for minimally-invasive
applications.
Please visit www.renovos.co.uk to learn more.